Research
1. Synthesis of Novel π-Extended Molecules Using Transition Metal Catalysts
In recent years, various novel functional organic molecules have been reported, which are directed toward active materials for organic devices. However, in order to create organic functional devices with truly practical performance, it is essential to synthesize more innovative molecules. For the purpose, thienoacenes, which are acene-based molecule containing a thiophene ring, are attracting considerable attention. We are focusing on novel thienoacenes, especially containing other heteroatoms, as candidates for functional organic molecules. Therefore, one of our interests is the development of new transition metal-catalyzed reactions as efficient tools to synthesize a variety of thienoacene derivatives (Figure 1).
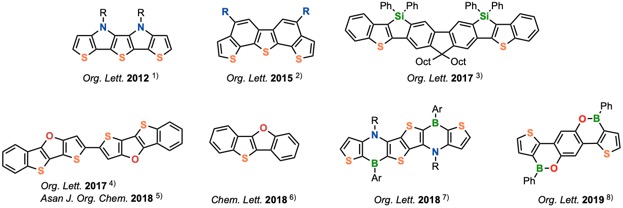
Figure 1. Our Synthesized Thienoacene Derivatives
2. Electrochemical Reactions Using Electro-generated Active Organometallic Species
Nowadays, electrochemical reactions have rapidly attracted attention. We have studied electrochemical reactions for more than fifteen years, and have developed several electrochemical reactions using electrochemically generated cationic palladium species. For example, we reported a Wacker-type reaction,9 a homo-coupling reaction of arylboronic acids,10 and an oxidative Sonogashira-type coupling reaction of arylboronic acids with alkynes11 (Figure 2). The generated active species are highly reactive and these reaction systems have a wide substrate scope than conventional chemical reactions.
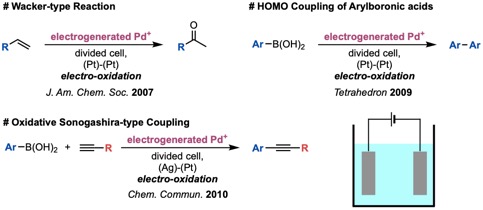
Figure 2. Electrochemical Reactions Using Electro-generated Pd Species
3. Synthesis of Heteroacenes via Electrochemical C–X Bond Formation
Recently, we have been working on the development of electrochemical C–X bond formations without the use of transition metals. For example, we reported a dehydrogenative reaction via S–H/C–H bond cleavage using an electrochemically generated [Br+] (Scheme 1).12 This reaction is the first synthesis of thienoacenes through electrochemical C–S bond formation.
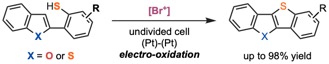
Scheme 1. Electrochemical Synthesis of Thienoacenes through a Dehydrogenative Coupling Using [Br+] as a Mediator
References
- Koichi Mitsudo,* Shuichi Shimohara, Jun Mizoguchi, Hiroki Mandai, Seiji Suga*
Org. Lett. 2012, 14, 2702–2705.
DOI: 10.1021/ol300887t - Koichi Mitsudo,* Hidehiko Sato, Arata Yamasaki, Natsuyo Kamimoto, Jun Goto, Hiroki Mandai, Seiji Suga*
Org. Lett. 2015, 17, 4858–4861.
DOI: 10.1021/acs.orglett.5b02417 - Koichi Mitsudo,* Seiichi Tanaka, Ryota Isobuchi, Tomohiro Inada, Hiroki Mandai, Toshinobu Korenaga, Atsushi Wakamiya, Yasujiro Murata, Seiji Suga*
Org. Lett. 2017, 19, 2564–2567.
DOI: 10.1021/acs.orglett.7b00878 - Koichi Mitsudo,* Yuji Kurimoto, Hiroki Mandai, Seiji Suga*
Org. Lett. 2017, 19, 2821–2824.
DOI: 10.1021/acs.orglett.7b00969 - Yuji Kurimoto, Koichi Mitsudo,* Hiroki Mandai, Atsushi Wakamiya, Yasujiro Murata, Hiroki Mori, Yasushi Nishihara, Seiji Suga*
Asian J. Org. Chem. 2018, 7, 1635–1641.
DOI: 10.1002/ajoc.201800270 - Koichi Mitsudo,* Takuya Asada, Tomohiro Inada, Yuji Kurimoto, Hiroki Mandai, Seiji Suga*
Chem. Lett. 2018, 47, 1044–1047.
DOI: 10.1246/cl.180425 - Koichi Mitsudo,* Keisuke Shigemori, Hiroki Mandai, Atsushi Wakamiya, Seiji Suga*
Org. Lett. 2018, 20, 7336–7340.
DOI: 10.1021/acs.orglett.8b03316 - Keisuke Shigemori, Momoka Watanabe, Julie Kong, Koichi Mitsudo,* Atsushi Wakamiya, Hiroki Mandai, Seiji Suga*
Org. Lett. 2019, 21, 2171–2175.
DOI: 10.1021/acs.orglett.9b00485 - Koichi Mitsudo,* Takashi Kaide, Eriko Nakamoto, KentaYoshida, Hideo Tanaka*
J. Am. Chem. Soc. 2007, 129, 2246–2247.
DOI: 10.1021/ja069043r - Koichi Mitsudo,* Takuya Shiraga, Daisuke Kagen, Deqing Shi, James Y. Becker, Hideo Tanaka*
Tetrahedron 2009, 65, 8384–8388.
DOI: 10.1016/j.tet.2009.08.004 - Koichi Mitsudo,* Takuya Shiraga, Jun-ichi Mizukawa, Seiji Suga, Hideo Tanaka*
Chem. Commun. 2010, 46, 9256–9258.
DOI: 10.1039/C0CC02633F - Koichi Mitsudo,* Ren Matsuo, Toki Yonezawa, Haruka Inoue, Hiroki Mandai, Seiji Suga*
Angew. Chem., Int. Ed. 2020, 59, 7803–7807.
DOI: 10.1002/anie.202001149





